Error Prevention at Sample Preparation on the AQUIOS CL System
The AQUIOS CL system employs several features that work to prevent errors during sample prep. These prevention methods can be broken down into the following:
Table 3. Error Prevention Sample Prep at a Glance
Flow Cell Clogging Prevention
Traditional flow cytometers use the prime and flush functions to mitigate flow cell clogs. In addition to the prime and flush functions used to mitigate flow cell clogs, the AQUIOS CL flow cytometer incorporates four built-in features to prevent clogs:
- A blunt probe. Using the specimen tube more than once can cause coring of the septa cap which can contaminate the sample and clog the flow cell. To minimize this from happening, the AQUIOS CL flow cytometer uses a blunt sample probe.
- 2 holes in the sample probe. Two holes in the sample probe reduces the probability of a probe clog.
- A filter before the flow cell. The filter works to keep large particles from reaching the flow cell.
- Real-time monitoring of the sample flow rate. The system monitors the sample flow rate for potential changes from partial clogs and bubbles and triggers an auto-repeat. Flow rate instability is measured by data rate and channel number. For example, if the channel is drifting, there is likely a clog in the system. The system also looks for insufficient data since an aperture clog could trigger this condition. When an auto-repeat is triggered, the system automatically aborts the run, runs a cleaning cycle, re-aspirates the sample from the 96-Deep Well Plate and re-analyzes the sample.
By combining these features with autocleaning (refer to Table 2), the AQUIOS CL flow cytometer minimizes the need to monitor the system for clogs and is part of what makes it a true Load & Go system.
Reducing Tandem Dye Degradation, Evaporation and Condensation
Unlike the emission spectra of most single fluorochromes, tandem dyes are known to display a great deal of variability in “fluorescence intensities within different regions of each emission spectrum.”3 The degradation of tandem dyes is largely due to light exposure that causes “photon-induced oxidation.”3 Degredation of tandem dyes can occur “within minutes of ambient light exposure.”3 When cell-labeling procedures involve large batch numbers or are conducted in “circumstances where light protective measures are difficult to maintain,” a large degree of variability and degradation are introduced3. Monoclonal antibodies used on traditional flow cytometers typically use amber or opaque white vials to eliminate light exposure. On these traditional flow cytometers, the vials are typically capped for transport and storage, but must be uncapped for preparation and analysis. It is well-known that sample preparation can introduce variability due to light exposure3. Many labs combat this potential for variability by conducting sample preparation activities in a dark laminar flow hood3.
The AQUIOS CL system uses similar methods to combat tandem dye degradation, but takes it one step further. Monoclonal antibodies are packaged in amber vials and remain capped throughout transport and storage just like traditional flow cytometers. But, since the AQUIOS monoclonal antibody caps are pierceable, this cap also remains on the vial during preparation and analysis further reducing the risk of light exposure. By keeping the cap on throughout the entire workflow, the AQUIOS CL system also reduces the risk of evaporation, condensation, and contamination. In addition, since sample preparation is done on the system in a closed environment, this further reduces the risk of degradation due to light exposure.
By reducing tandem dye degradation to a point where spectral compensation is minimally affected, the AQUIOS CL system is able to implement “predetermined compensation matrices and analysis templates with preset regions and gating strategies.” This simplified approach is ideal for a clinical setting as long as the cell populations meet the system’s required separation3. With the innovative “bottom-up” gating strategy that the AQUIOS CL system employs, you can rely on good isolation of the desired populations. Refer to the AQUIOS Tetra Application Gating Strategy product bulletin for more information on the gating strategies used on the AQUIOS CL system.
Absolute Counting
Traditional flow cytometers are not volumetric; instead, they rely on fluorospheres for accurate counts. The beads have a specific concentration. These instruments take the concentration plus the known volume added to the sample and calculate the count based on the 1:1 dilution. But, if there is any deviation in the volume of either blood or beads, this can skew the counts.
Volumetric absolute counting is a method used on the AQUIOS CL flow cytometer Tetra Tests that uses calibrated syringes to deliver a specific amount of fluid volume within a specific time. The system is calibrated upon installation by a Beckman Coulter Representative. Once calibrated, the system automatically determines the absolute count.
One of the benefits of this method is that it eliminates the need for daughter tubes and pipetting, both of which have known risks associated with mislabeling, misidentification, and pipetting errors.
Sample Mixing
If using the autoloader, the cassette with the samples is rocked prior to cap-piercing each of the tubes. Simply load the cassettes with the samples in the autoloader. Samples for the Single-tube Loader must be mixed by the user prior to loading the sample.
Sample Prep
When sample preparation is not standardized, it increases the risk of error. Sample misidentification and time are the two most critical variables when dealing with the disconnect in the process between a sample prep system and a flow cytometer. The need to manually transfer samples between the systems leaves room for misidentification errors including mislabeling of daughter tubes, and misplacement of samples. The AQUIOS CL eliminates the risk of misidentification by incorporating the sample prep process directly into the flow cytometry system. Sample preparation also supports various tasks that rely on consistent time windows. For example, if you batch 40 samples, the timing on a typical flow cytometer is not consistent. On a traditional flow cytometer, since sample preparation is done outside of the system, sample 1 is processed immediately, but sample 40 remains in the queue on the system until all of the other samples are processed. This allows for an inconsistency in the preparation time to processing time. The user is responsible for monitoring this. There is no automatic notification if any step in the process is beyond the time limit.
The AQUIOS CL flow cytometer integrates sample prep directly into the system; samples are aliquotted, stained, incubated and lysed onboard. The AQUIOS CL flow cytometer, therefore, treats every sample the same way. A software scheduler ensures that all samples are prepared and analyzed within the required time. Since sample preparation is done on the system, the instrument is able to schedule preparation times to keep the time variable between sample preparation and sample processing consistent. As the first sample is incubating, the second sample is preparing. Every step has a predefined time window which eliminates time differences that can lead to under and over lysing and inconsistent sample suspension due to settling. Refer to Figure 3 for the sample preparation stations.
Figure 3. AQUIOS CL Flow Cytometer Sample-Preparation Stations
Daughter Tubes
The AQUIOS CL system continually tracks where samples are in the process eliminating the need to manually track daughter tubes. Specifically, the system provides traceability between the specimen ID and the position of the prepared sample reducing the possibility for sample misidentification.
Blood Aspiration
Blood Aspiration Detection
On the AQUIOS CL flow cytometer, there is a sensor on the instrument that uses spectrophotometry to determine the difference between blood, air, and sheath fluid based on color. The system uses this sensor as a means to determine if blood is actually aspirating. The system displays a short draw flag to notify the user if a sufficient amount of blood was not aspirated.
Specimen Tube Vacuum/Pressure
The AQUIOS CL flow cytometer borrows the concept of an aspiration buffer from Hematology as a means of negating the impact of vacuum and pressure on aspiration. On other flow cytometry instruments, users would account for the impact of varying vacuum and pressure by venting the tubes before aspiration if the specimen tube was previously opened. This is no longer necessary on the AQUIOS CL system.
Fill the form to download Workflow Comparison Study with AQUIOS CL Flow Cytometer

HIV Advanced Disease Management

HIV Advanced Disease Management Solutions
Monitoring CD4 lymphocyte counts is essential in providing critical information that impacts patient care. CD4 monitoring allows caregivers to know when the disease transforms and stage its progression so they can implement the most appropriate intervention..
Fast Throughput Subset Analysis with AQUIOS CL
In the clinical management of immune deficiency diseases, accurately counting the absolute cell numbers of leukocyte subsets and measuring the percentage of individual subtypes in blood is critical.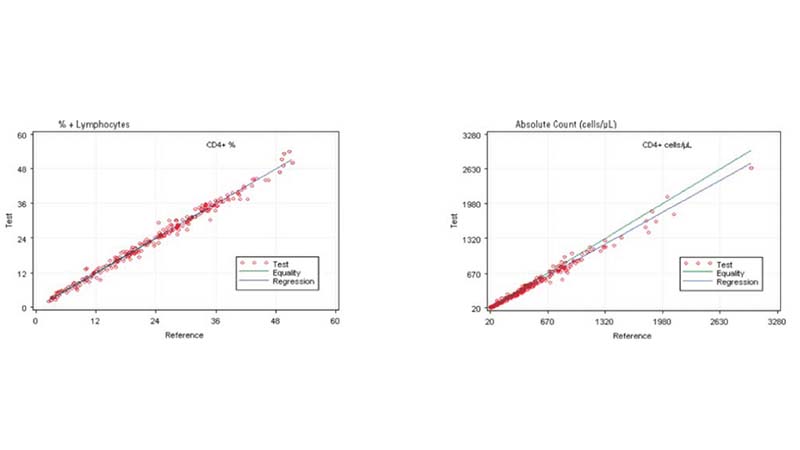
Comparing the AQUIOS CL PLG Application to the FC500 MCL FlowCARE PLG Application
Based on this study, the data suggests a strong correlation between the AQUIOS CL PLG application and the FC500 MCL FlowCARE PLG application.
AQUIOS CL Tetra and Aged Whole Blood samples
Based on this study, the AQUIOS CL instrument with Tetra application provides accurate results for recovery of the T, B and NK lymphocyte subsets in clinical and normal specimens collected into the EDTA K3 tubes when stored at room temperature for up to 24 hours.Error Prevention on AQUIOS CL overview
Laboratory standards and regulations set forth by governmental bodies such as the Centers for Disease Control (CDC) help to define the procedures that laboratories follow
Error prevention during Startup and Worklist creation
The AQUIOS CL system employs several features that work to prevent errors during startup, cleaning, and worklist generation, including Autocleaning, Test Requests to and from the LIS and Creating Worklists.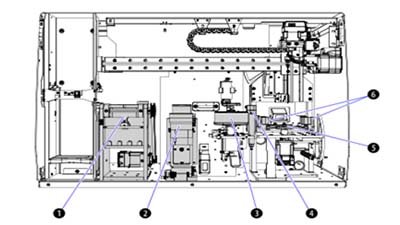
Error Prevention During Sample Prep
The AQUIOS CL system employs several features that work to prevent errors during sample prep.
Error Prevention During Quality Control
The AQUIOS CL system employs several features that work to prevent errors during quality control, including Automatically Pause when QC Fails, QC Checks, Daily QC (Controls), Real Time QC (Patient Sample).References:
- 1997 Revised Guidelines for the Performance of CD4+ T-Cell Determination in Persons with Human Immunodeficiency Virus (HIV) Infection. MMWR, January 10, 1997, p. 11.
- Levey S., Jennings, E.R., (1950) The use of control charts in the clinical laboratory. Am. J. Clin. Pathol. 20:1059.
- Hulspas R, Dombkowski D, Preffer F, Douglas D, Kildew-Shah B, Gilbert J. Flow Cytometry and the Stability of Phycoerythrin-Tandem Dye Conjugates. International Society for Advancement of Cytometry. September 23, 2009.
- Hulspas R, O’Gorman MRG, Wood BL, Gratama, JW, Sutherland DR. Considerations for the control of background fluorescence in clinical flow cytometry. Cytometry Part B 2009;76B:355-364.
- Centers for Disease Control and Prevention. 2003 Guidelines for performing single-platform absolute CD4+ T-cell determinations with CD45 gating for persons infected with Human Immunodeficiency Virus HIV). MMWR 2003; 57 (No. RR. 2): 1-13.


